Benzene and its Derivatives Notes (Organic Chemistry 2)
Download comprehensive handwritten notes on Benzene and its Derivatives, covering Unit 1 of Organic Chemistry 2. These notes are perfect for students looking for detailed explanations and easy-to-understand content. Access them as a PDF or view directly online for free.
Keywords: benzene derivatives notes, organic chemistry notes, unit 1, handwritten notes, benzene structure, Kekule, resonance, Huckel rules, electrophilic substitution, nitration, halogenation, Friedel-Crafts, DDT, BHC, Chloramine, download PDF, free study material, chemistry notes.
Benzene and its Derivatives: A Comprehensive Study (Unit 1, Organic Chemistry 2)
This detailed set of handwritten notes delves into the fascinating world of Benzene and its various derivatives, forming a crucial part of Unit 1 in Organic Chemistry 2. Designed for clarity and comprehensive understanding, these notes cover everything from the fundamental structure of benzene to complex reaction mechanisms and the properties of significant derivatives like DDT, BHC, and Chloramine.
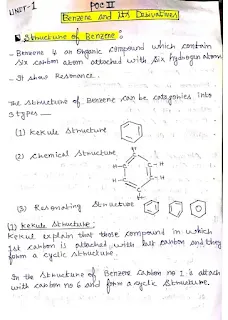
Understanding Benzene's Unique Structure
The notes begin by exploring the multifaceted structure of benzene. You'll gain insights into its chemical representation, the historical Kekulé structure, and the more accurate resonance structures that explain its unusual stability. Beyond these fundamental aspects, the content also touches upon analytical, synthetic, and other advanced structural models, providing a holistic view of this iconic aromatic compound. The orbital picture of benzene is meticulously explained, helping you visualize the delocalized pi-electron system that underpins its aromaticity.
Aromaticity and Huckel's Rule
A significant portion is dedicated to the concept of resonance in benzene, illustrating how electron delocalization contributes to its stability. The notes thoroughly explain aromatic characteristics and the application of Hückel's Rule (4n+2 rule), which is essential for determining the aromatic nature of cyclic, planar molecules. Understanding these principles is key to predicting the reactivity of benzene and its analogs.
Reactions of Benzene: Electrophilic Aromatic Substitution
The core reactivity of benzene is dominated by electrophilic aromatic substitution (EAS) reactions. These notes provide an in-depth analysis of various EAS reactions, including:
- Nitration: The introduction of a nitro group onto the benzene ring.
- Sulfonation: The addition of a sulfonic acid group.
- Halogenation: The substitution of a hydrogen atom with a halogen (e.g., chlorination, bromination), detailing reactivity patterns.
- Friedel-Crafts Alkylation: The attachment of an alkyl group, along with important limitations of this reaction.
- Friedel-Crafts Acylation: The introduction of an acyl group, often preferred over alkylation due to fewer limitations.
The section on "Effect of Substituents" is critical, explaining how groups already present on the benzene ring influence the rate and regioselectivity of subsequent electrophilic substitutions (ortho, meta, para directing effects).
Key Benzene Derivatives: DDT, BHC, and Chloramine
Finally, the notes extend to provide detailed information on several important benzene derivatives, including their uses, properties, structures, and preparation methods:
- DDT (Dichlorodiphenyltrichloroethane): A historically significant insecticide, its structure, synthesis, properties, and applications are discussed.
- BHC (Benzene Hexachloride): Another important insecticide, with a focus on its different isomers, properties, and uses.
- Chloramine: Explaining its structure, properties, and applications, particularly in water treatment and as a disinfectant.
These comprehensive handwritten notes serve as an invaluable resource for students of Organic Chemistry 2, offering clarity, depth, and all the essential information needed to master Benzene and its derivatives.
Info!
If you are the copyright owner of this document and want to report it, please visit the copyright infringement notice page to submit a report.